COVID-19: Oxford/AstraZeneca vaccine approved for use in UK
The Oxford/AstraZeneca vaccine has become the second coronavirus jab to be approved for UK use.
It has been given the go-ahead by the Medicines and Healthcare products Regulatory Agency (MHRA).
The vaccine, codenamed AZD1222, was developed at Oxford University with support from the British-Swedish pharmaceutical giant AstraZeneca.
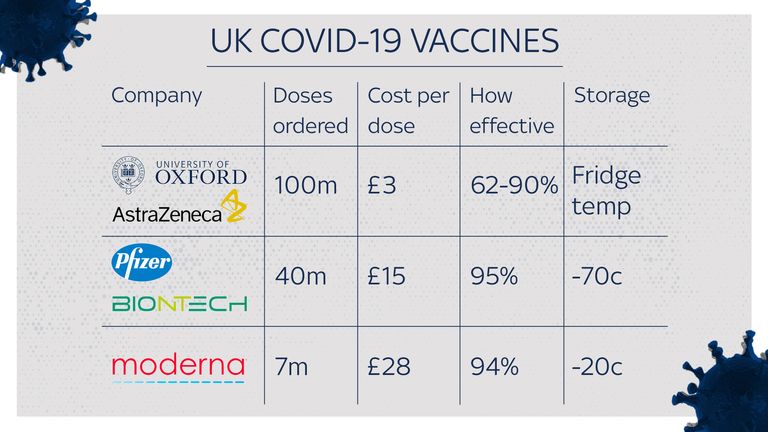
Results from clinical trials showed that it is up to 90% effective in preventing COVID-19, almost matching the protection by the rival Pfizer/BioNTech and Moderna jabs.
Health Secretary Matt Hancock told Sky News the Oxford vaccine will start to be rolled from 4 January and that it will help “accelerate” vaccinating the nation.
He said: “It’s very good news for accelerating the vaccine rollout. It brings forward the day we can get our lives back to normal.
“The vaccine is our way out of the pandemic.”
Independent analysis published in The Lancet also confirmed the Oxford/AstraZeneca vaccine is safe and effective after looking into phase 3 data of a vaccine trial.
Once regarded as the frontrunner in the COVID vaccine race, the British team were overtaken by US drugmaker Pfizer with a success rate of 95%, with that jab already being rolled out in the UK.
While the vast majority of the 100 million doses of the drug ordered by the UK from AstraZeneca will be made in the UK, giving the Oxford vaccine a considerable advantage over its rivals, the initial rollout will use doses manufactured in Europe.
Unlike the Pfizer vaccine, the Oxford vaccine does not need to be stored at ultra-low temperatures.
Like most other vaccines, it will need to be sent to vaccination centres in refrigerated vans or cool boxes and kept in a special vaccine fridge between 2C to 8C, and protected from light.
It is also the cheapest of all the COVID vaccines, costing “the same as a cup of coffee”, as Sky News understands it will be a little under £3 per dose, with one and a half or two doses required.
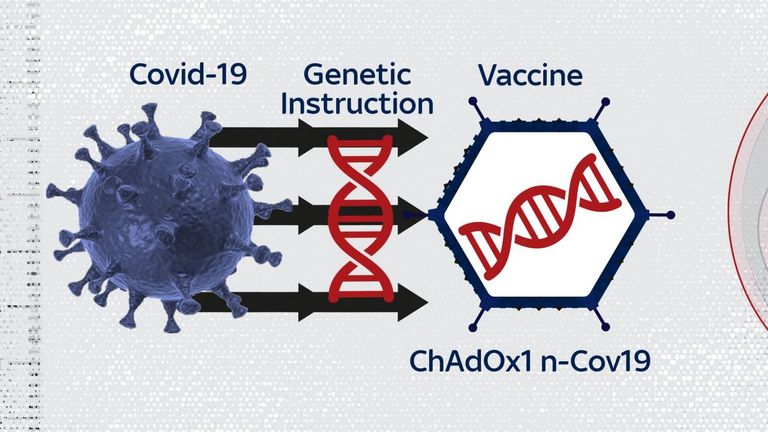
The vaccine is based on an adenovirus, which causes the common cold in chimpanzees.
The virus is modified so it can’t cause disease in humans. It also carries an extra gene from the coronavirus.
Inside the human body, the gene makes the spike protein that coats the shell of the coronavirus, stimulating an immune response that protects against infection.
For one course of dosing – where people were given a half dose of the Oxford vaccine, followed by a full measure at least a month after – there was an efficacy rate of 90%.
That fell to an efficacy of 62% when two full doses were given at least a month apart.
A total of 2,741 people were on the course that proved 90% effective, while 8,895 were given two full doses.
When all the results are tabulated, the average efficacy of the vaccine worked out to 70%.
Source: Read Full Article